Unit 3 Equilibrium
3.1 Microscopic and Macroscopic Aspects of Equilibrium
OpenStax
Section Learning Objectives
- Describe the nature of equilibrium systems.
- Explain the dynamic nature of a chemical equilibrium.
✓ SECTION 3.1 CHECKLIST
| Learning Activity | Graded? | Estimated Time |
|---|---|---|
| Make notes about this section’s reading portion. | No | 30 min |
| Optional Resource: Watch the suggested video. | No | 6 min |
| Work on the self-check question. | No | 10 min |
| Work on practice exercises. | No | 10 min |
📖 READING PORTION
The Concept of Equilibrium
The convention for writing chemical equations involves placing reactant formulas on the left side of a reaction arrow and product formulas on the right side. By this convention, and the definitions of “reactant” and “product,” a chemical equation represents the reaction in question as proceeding from left to right. Reversible reactions, however, may proceed in both forward (left to right) and reverse (right to left) directions. When the rates of the forward and reverse reactions are equal, the concentrations of the reactant and product species remain constant over time and the system is at equilibrium. The relative concentrations of reactants and products in equilibrium systems vary greatly; some systems contain mostly products at equilibrium, some contain mostly reactants, and some contain appreciable amounts of both. Among all these equilibrium systems, the commonality is that there is no net change in reactant or product concentration over time.
For equilibrium systems, a special double arrow ⇌ is used to emphasize the reversible nature of the reaction. Figure 1 illustrates fundamental equilibrium concepts using the reversible decomposition of colorless dinitrogen tetroxide to yield brown nitrogen dioxide, an elementary reaction described by the equation
N2O4(g) ⇌ 2 NO2(g)
For this elementary process, rate laws for the forward and reverse reactions may be derived directly from the reaction stoichiometry
ratef = kf[N2O4]
rater = kr[NO2]2
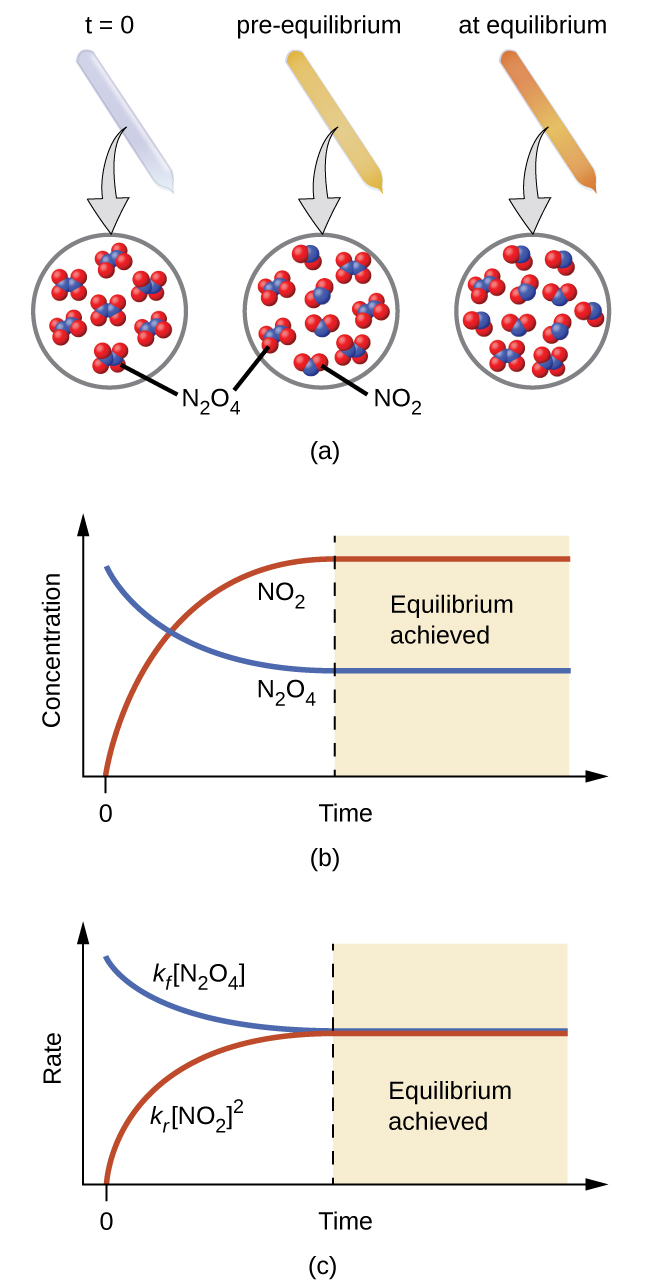
As the reaction begins (t = 0), the concentration of the N2O4 reactant is finite and that of the NO2 product is zero, so the forward reaction proceeds at a finite rate while the reverse reaction rate is zero. As time passes, N2O4 is consumed and its concentration falls, while NO2 is produced and its concentration increases (Figure 1(b)). The decreasing concentration of the reactant slows the forward reaction rate, and the increasing product concentration speeds the reverse reaction rate (Figure 1(c)). This process continues until the forward and reverse reaction rates become equal, at which time the reaction has reached equilibrium, as characterized by constant concentrations of its reactants and products (shaded areas of Figure 1(b) and Figure 1(c)). It’s important to emphasize that chemical equilibria are dynamic; a reaction at equilibrium has not “stopped”. The forward and reverse reaction rates are equal at equilibrium, but the reactant and product concentrations are not necessarily equal.
Physical changes, such as phase transitions, are also reversible and may establish equilibria. As one example, consider the vaporization of bromine
Br2(l) ⇌ Br2(g)
When liquid bromine is added to an otherwise empty container and the container is sealed, the forward process (vaporization) will commence and continue at a roughly constant rate as long as the exposed surface area of the liquid and its temperature remain constant. As increasing amounts of gaseous bromine are produced, the rate of the reverse process (condensation) will increase until it equals the rate of vaporization and equilibrium is established. A photograph showing this phase transition equilibrium is provided in Figure 2.

Optional Resource
Watch a video explanation about equilibrium.
Video 1 GCSE Chemistry – Reversible Reactions and Equilibrium ( min).
At equilibrium, the rate of the forward reaction equals the rate of the reverse reaction. True or false?
Click to see answer
True

