Unit 2 Kinetics
2.6 Catalysis
OpenStax
Section Learning Objectives
- Explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams.
- List examples of catalysis in natural and industrial processes.
✓ SECTION 2.6 CHECKLIST
| Learning Activity | Graded? | Estimated Time |
|---|---|---|
| Make notes about this section’s reading portion. | No | 90 min |
| Optional Resource: Read about catalytic converters. | No | 30 min |
| Optional Resource: Watch a video explanation about catalysis. | No | 2 min |
| Work on the self-check question. | No | 15 min |
| Work on practice exercises. | No | 90 min |
| Complete Unit 2 Assignment on Moodle. | Yes | 180 min |
📖 READING PORTION
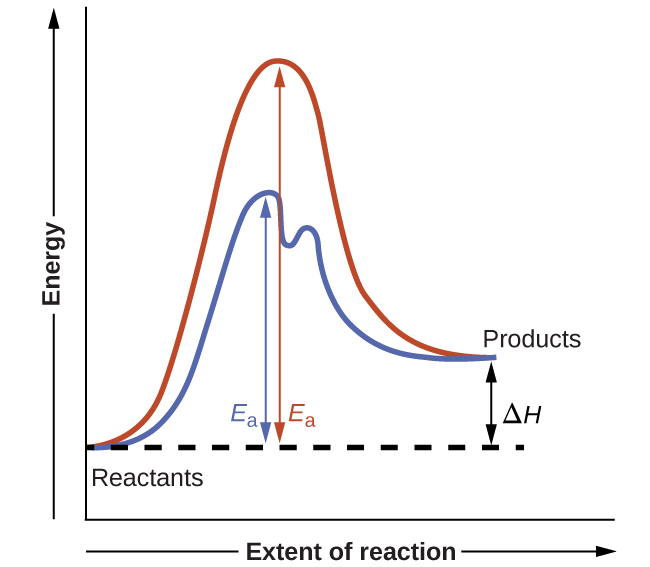
Among the factors affecting chemical reaction rates discussed earlier in this Unit was the presence of a catalyst, a substance that can increase the reaction rate without being consumed in the reaction. The concepts introduced in the previous section on reaction mechanisms provide the basis for understanding how catalysts are able to accomplish this very important function.
Figure 1 shows potential energy diagrams for a chemical process in the absence and presence of a catalyst. Inspection of the diagrams reveals several traits of these reactions. Consistent with the fact that the two diagrams represent the same overall reaction, both curves begin and end at the same energies (in this case, because products are more energetic than reactants, the reaction is endothermic). The reaction mechanisms, however, are clearly different. The uncatalyzed reaction proceeds via a one-step mechanism (one transition state observed), whereas the catalyzed reaction follows a two-step mechanism (two transition states observed) with a notably lesser activation energy. This difference illustrates the means by which a catalyst functions to accelerate reactions, namely, by providing an alternative reaction mechanism with a lower activation energy. Although the catalyzed reaction mechanism for a reaction need not necessarily involve a different number of steps than the uncatalyzed mechanism, it must provide a reaction path whose rate determining step is faster (lower Ea).
Homogeneous Catalysts
A homogeneous catalyst is present in the same phase as the reactants. It interacts with a reactant to form an intermediate substance, which then decomposes or reacts with another reactant in one or more steps to regenerate the original catalyst and form product.
As an important illustration of homogeneous catalysis, consider the earth’s ozone layer. Ozone in the upper atmosphere, which protects the earth from ultraviolet radiation, is formed when oxygen molecules absorb ultraviolet light and undergo the reaction
3 O2(g) → 2 O3(g)
Ozone is a relatively unstable molecule that decomposes to yield diatomic oxygen by the reverse of this equation. This decomposition reaction is consistent with the following two-step mechanism
O3 ⟶ O2 + O
O3 + O ⟶ 2 O2
A number of substances can catalyze the decomposition of ozone. For example, the nitric oxide–catalyzed decomposition of ozone is believed to occur via the following three-step mechanism
NO(g) + O3(g) ⟶ NO2(g) + O2(g)
O3(g) ⟶ O2(g) + O(g)
NO2(g) + O(g) ⟶ NO(g) + O2(g)
As required, the overall reaction is the same for both the two-step uncatalyzed mechanism and the three-step NO-catalyzed mechanism
2 O3(g) ⟶ 3 O2(g)
Notice that NO is a reactant in the first step of the mechanism and a product in the last step. This is another characteristic trait of a catalyst: Though it participates in the chemical reaction, it is not consumed by the reaction.
Heterogeneous Catalysts
A heterogeneous catalyst is a catalyst that is present in a different phase (usually a solid) than the reactants. Such catalysts generally function by furnishing an active surface upon which a reaction can occur. Gas and liquid phase reactions catalyzed by heterogeneous catalysts occur on the surface of the catalyst rather than within the gas or liquid phase. Gases reacting on a solid catalytic surface may be zeroth-order.
Heterogeneous catalysis typically involves the following processes:
- Adsorption of the reactant(s) onto the surface of the catalyst.
- Activation of the adsorbed reactant(s).
- Reaction of the adsorbed reactant(s).
- Desorption of product(s) from the surface of the catalyst.
Figure 2 illustrates the steps of a mechanism for the reaction of compounds containing a carbon–carbon double bond with hydrogen on a nickel catalyst. Nickel is the catalyst used in the hydrogenation of polyunsaturated fats and oils (which contain several carbon–carbon double bonds) to produce saturated fats and oils (which contain only carbon–carbon single bonds).
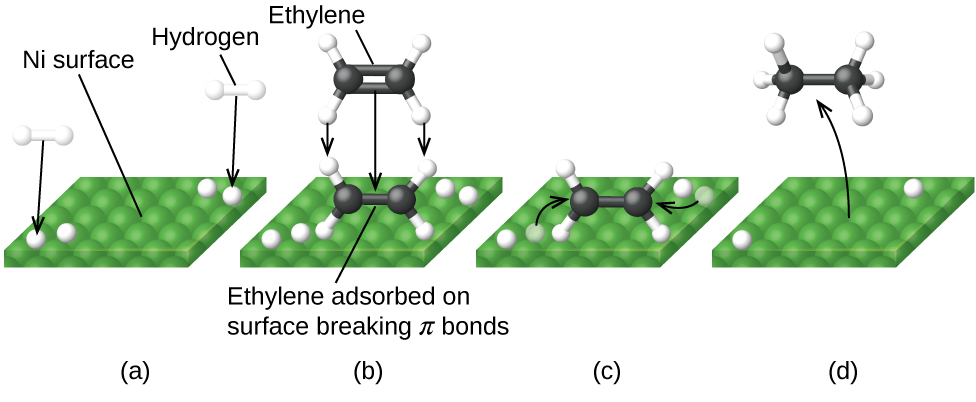
Many important chemical products are prepared via industrial processes that use heterogeneous catalysts, including ammonia, nitric acid, sulfuric acid, and methanol. Heterogeneous catalysts are also used in the catalytic converters found on most gasoline-powered automobiles
Enzyme Structure and Function
The study of enzymes is an important interconnection between biology and chemistry. Enzymes are usually proteins (polypeptides) that help to control the rate of chemical reactions between biologically important compounds, particularly those that are involved in cellular metabolism. It is possible for enzymatic reactions to be zeroth-order.
Enzyme molecules possess an active site, a part of the molecule with a shape that allows it to bond to a specific substrate (a reactant molecule), forming an enzyme-substrate complex as a reaction intermediate. There are two models that attempt to explain how this active site works. The most simplistic model is referred to as the lock-and-key hypothesis, which suggests that the molecular shapes of the active site and substrate are complementary, fitting together like a key in a lock (Figure 3(a)). The induced fit hypothesis, on the other hand, suggests that the enzyme molecule is flexible and changes shape to accommodate a bond with the substrate (Figure 3(b)). This is not to suggest that an enzyme’s active site is completely malleable, however. Both the lock-and-key model and the induced fit model account for the fact that enzymes can only bind with specific substrates, since in general a particular enzyme only catalyzes a particular reaction.

Optional Resources
Read about catalytic converters. The University of California at Davis’ “ChemWiki” provides a thorough explanation of how catalytic converters work.
Section 2.5, Video 1 provides an example about how to identify the homogeneous catalyst in a reaction mechanism.
Watch the video explanation about catalysis.
Video 1 Catalysis (1 min 15 s).
Which of the following is false?
A) Catalysts are important for industrial productivity.
B) Nickel is a heterogeneous catalyst in the food industry.
C) Catalysts are not listed as reactants in the net equation.
D) A catalyst needs to be supplied constantly to the reaction.
Click to see answer
D) A catalyst needs to be supplied constantly to the reaction.
✩ UNIT 2 ASSIGNMENT
Complete Unit 2 Assignment (graded) on Moodle. This assignment is worth 11% of the course grade.

