Unit 2 Kinetics
2.5 Reaction Mechanisms
OpenStax
Section Learning Objectives
- Distinguish net reactions from elementary reactions (steps).
- Identify the molecularity of elementary reactions.
- Write a balanced chemical equation for a process given its reaction mechanism.
- Derive the rate law consistent with a given reaction mechanism.
✓ SECTION 2.5 CHECKLIST
| Learning Activity | Graded? | Estimated Time |
|---|---|---|
| Make notes about this section’s reading portion. | No | 90 min |
| Optional Resource: Watch a video explanation of reaction mechanisms. | No | 19 min |
| Work on the self-check question. | No | 15 min |
| Work on practice exercises. |
No | 60 min |
📖 READING PORTION
The Individual Steps that Make Up a Chemical Reaction
Chemical reactions very often occur in a step-wise fashion, involving two or more distinct reactions taking place in sequence. A balanced net equation indicates what is reacting and what is produced, but it reveals no details about how the reaction actually takes place. The reaction mechanism (or reaction path) provides details regarding the precise, step-by-step process by which a reaction occurs.
Consider the net reaction
C4H9Br + H2O ⟶ C4H8 + H3O+ + Br−
which occurs via the mechanism
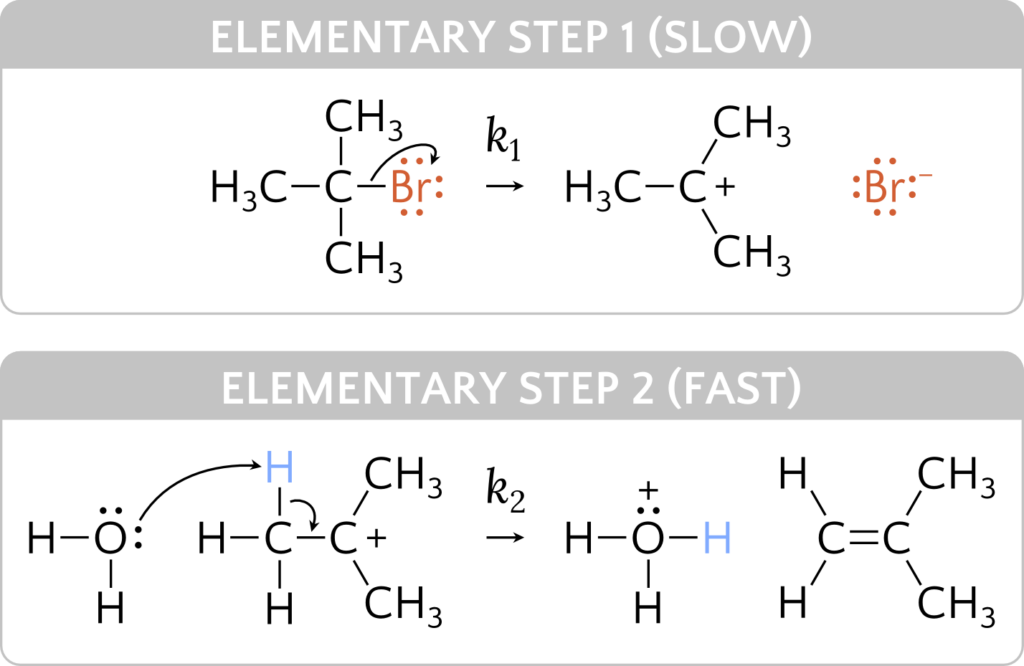
The steps of a reaction mechanism sum to the net reaction. The species C4H9+ is called the intermediate and does not appear in the net reaction because the intermediate is produced and consumed within the elementary steps. There are other noteworthy features about the steps of a reaction mechanism.
The molecularity of an elementary reaction is the number of reactant species (atoms, molecules, or ions). For example, a unimolecular reaction involves the fragmentation of a single reactant species to produce one or more molecules of product. A bimolecular reaction involves a collision of two reactant species. The first step in the reaction mechanism is unimolecular, where a C4H9Br molecule is the sole reacting species. The second step is bimolecular, where C4H9+ and H2O react together. An elementary termolecular reaction involves the simultaneous collision of three atoms, molecules, or ions. Termolecular elementary reactions are uncommon because the probability of three particles colliding simultaneously is less than one one-thousandth of the probability of two particles colliding. There are, however, a few established termolecular elementary reactions. The reaction of nitric oxide with oxygen appears to involve termolecular steps
2 NO + O2 ⟶ 2 NO2
Likewise, the reaction of nitric oxide with chlorine appears to involve termolecular steps
2 NO + Cl2 ⟶ 2 NOCl
Elementary reactions may have different rates as some steps may be faster or slower than others. Each step has a rate constant k. The slowest step is the rate-determining step of the overall reaction. Because a reaction cannot proceed faster than its slowest step, this step will limit the rate at which the overall reaction occurs. The rate law is written based on the rate-determining (slowest) step of the reaction mechanism. The reactant coefficients on the rate-determining step become the exponents on the product concentrations in the rate law. In the above reaction mechanism, the rate law is therefore
rate = k1[C4H9Br]
If the rate-determining step contains intermediate species, these intermediate species in the rate law will need to be rewritten using the species in the net reaction. Replacement of intermediate species is beyond the scope of CHEM 1523.
Unlike balanced equations representing an overall reaction, the equations for elementary reactions are explicit representations of the chemical change taking place. The reactant(s) in an elementary reaction’s equation undergo only the bond-breaking and/or making events depicted to yield the product(s). For this reason, the rate law for an elementary reaction may be derived directly from the balanced chemical equation describing the reaction. This is not the case for typical chemical reactions, for which rate laws may be reliably determined only via experimentation.
The reaction between C4H9Br and H2O is plotted on a potential energy diagram in Figure 1. The reactants, products, and enthalpy change are similar to what was presented in Section 2.4. The difference here is the presence of the two peaks in energy corresponding to the two elementary steps of the reaction mechanism, where the transition state of each step is located at the top of the peak. The intermediate C4H9+ is found in the “valley” between the two elementary steps. The relative rates of the two elementary steps correspond to the relative magnitudes of the activation energies Ea1 and Ea2. Elementary step 1 has a greater activation energy than step 2 and is slower than step 2. The higher the activation energy, the slower the elementary step. The step that has the highest activation energy is the slowest, rate-determining step of the reaction mechanism.

Figure 1 The reaction between C4H9Br and H2O is plotted on a potential energy diagram. The transition states are marked as TS1 and TS2 for the two elementary steps of the reaction mechanism. Source: Jung-Lynn Jonathan Yang
A reaction mechanism is consistent when the steps sum to the stoichiometry of the net equation and agree with the species and their orders in the rate law. The reaction of NO2 and CO provides an illustrative example
NO2(g) + CO(g) ⟶ CO2(g)+ NO(g)
For temperatures above 225°C, the rate law has been found to be
rate = k[NO2][CO]
The reaction is first-order with respect to NO2 and first-order with respect to CO. This is consistent with a single-step bimolecular mechanism and it is possible that this is the mechanism for this reaction at high temperatures. At temperatures below 225°C, the reaction is described by a rate law that is second-order with respect to NO2:
rate = k[NO2]2
This rate law is not consistent with the single-step mechanism, but is consistent with the following two-step mechanism
NO2(g) + NO2(g) ⟶ NO3(g) + NO(g) (slow)
NO3(g) + CO(g) ⟶ NO2(g) + CO2(g) (fast)
The rate-determining (slower) step gives a rate law showing second-order dependence on the NO2 concentration, and the sum of the two equations gives the net overall reaction.
The situation is more complicated when the rate-determining step is the second or later step in a reaction mechanism and the rate-determining step has intermediate species as reactant(s). In Section 2.5 Exercises, question 4, there is an intermediate species in the second step, which is also the rate-determining step. One way to solve this question is to eliminate the intermediate by adding the first step (fast step) to the second step (rate-determining step). A more sophisticated method to eliminate intermediate species in higher level chemistry courses is to treat the first step as an equilibrium reaction.
Optional Resource
Watch a video explanation using reaction mechanisms to write the rate law.
Video 1 Writing Rate Laws of Reaction Mechanisms Using The Rate Determining Step – Chemical Kinetics (18 min 47 s).
Consider the reaction:
mechanismCl(g) + O3(g) ⟶ ClO(g) + O2(g) (rate constant k1)
ClO(g) + O(g) ⟶ Cl(g) + O2(g) (rate constant k2)
Which species is the intermediate?
Click to see answer
ClO is the intermediate because ClO is produced and consumed in elementary steps.

