Unit 5 Thermodynamics
5.6 Exercises
OpenStax
Section 5.6 Exercises
- A reaction has [latex]{\Delta}H_{\text{r, }298}^{\circ} = 100\;\text{kJ/mol}[/latex] and [latex]{\Delta}S_{\text{r, 298}}^{\circ} = 250\;\text{J/(mol}{\cdot}\text{K)}[/latex]. Is the reaction spontaneous under standard conditions? If not, under what temperature conditions will it become spontaneous under standard conditions?
- Use the standard free energy of formation data (Source: OpenStax Chemistry 2e) to determine the free energy change for each of the following reactions, which are run under standard state conditions and 25 °C. Identify each as either spontaneous or nonspontaneous at these conditions.
(a) [latex]\text{MnO}_2(s)\;{\longrightarrow}\;\text{Mn}(s)\;+\;\text{O}_2(g)[/latex]
(b) [latex]\text{H}_2(g)\;+\;\text{Br}_2(l)\;{\longrightarrow}\;2\text{HBr}(g)[/latex]
(c) [latex]\text{Cu}(s)\;+\;\text{S}(g)\;{\longrightarrow}\;\text{CuS}(s)[/latex]
(d) [latex]2\text{LiOH}(s)\;+\;\text{CO}_2(g)\;{\longrightarrow}\;\text{Li}_2\text{CO}_3(s)\;+\;\text{H}_2\text{O}(g)[/latex]
(e) [latex]\text{CH}_4(g)\;+\;\text{O}_2(g)\;{\longrightarrow}\;\text{C}(s\text{, graphite})\;+\;2\text{H}_2\text{O}(g)[/latex]
(f) [latex]\text{CS}_2(g)\;+\;3\text{Cl}_2(g)\;{\longrightarrow}\;\text{CCl}_4(g)\;+\;\text{S}_2\text{Cl}_2(g)[/latex]
- Given:
[latex]\begin{array}{ll} \text{P}_4(s)\;+\;5\text{O}_2(g)\;{\longrightarrow}\;\text{P}_4\text{O}_{10}(s) & {\Delta}G_{\text{r, 298}}^{\circ} = -2697.0\;\text{kJ/mol} \\[0.5em] 2\text{H}_2(g)\;+\;\text{O}_2(g)\;{\longrightarrow}\;2\text{H}_2\text{O}(g) & {\Delta}G_{\text{r, 298}}^{\circ} = -457.18\;\text{kJ/mol} \\[0.5em] 6\text{H}_2\text{O}(g)\;+\;\text{P}_4\text{O}_{10}(g)\;{\longrightarrow}\;4\text{H}_3\text{PO}_4(l) & {\Delta}G_{\text{r, 298}}^{\circ} = -428.66\;\text{kJ/mol} \end{array}[/latex](a) Determine the standard free energy of formation, [latex]{\Delta}G_{\text{f}}^{\circ}[/latex], for phosphoric acid.
(b) How does your calculated result compare to the value here (Source: OpenStax Chemistry 2e)? Explain.
- Consider the decomposition of red mercury (II) oxide under standard state conditions.
[latex]2\text{HgO}(s\text{, red})\;{\longrightarrow}\;2\text{Hg}(l)\;+\;\text{O}_2(g)[/latex](a) Is the decomposition spontaneous under standard state conditions?
(b) Above what temperature does the reaction become spontaneous?
- Calculate ΔG° for each of the following reactions from the equilibrium constant at the temperature given.
(a) [latex]\text{N}_2(g)\;+\;\text{O}_2(g)\;{\longrightarrow}\;2\text{NO}(g)\;\;\;\;\;\;\;T = 2000\;^{\circ}\text{C}\;\;\;\;\;\;\;K_{\text{p}} = 4.1\;\times\;10^{-4}[/latex]
(b) [latex]\text{H}_2(g)\;+\;\text{I}_2(g)\;{\longrightarrow}\;2\text{HI}(g)\;\;\;\;\;\;\;T = 400\;^{\circ}\text{C}\;\;\;\;\;\;\;K_{\text{p}} = 50.0[/latex]
(c) [latex]\text{CO}_2(g)\;+\;\text{H}_2(g)\;{\longrightarrow}\;\text{CO}(g)\;+\;\text{H}_2\text{O}(g)\;\;\;\;\;\;\;T = 980\;^{\circ}\text{C}\;\;\;\;\;\;\;K_{\text{p}} = 1.67[/latex]
(d) [latex]\text{CaCO}_3(s)\;{\longrightarrow}\;\text{CaO}(s)\;+\;\text{CO}_2(g)\;\;\;\;\;\;\;T = 900\;^{\circ}\text{C}\;\;\;\;\;\;\;K_{\text{p}} = 1.04[/latex]
(e) [latex]\text{HF}(aq)\;+\;\text{H}_2\text{O}(l)\;{\longrightarrow}\;\text{H}_3\text{O}^{+}(aq)\;+\;\text{F}^{-}(aq)\;\;\;\;\;\;\;T = 25\;^{\circ}\text{C}\;\;\;\;\;\;\;K_{\text{p}} = 7.2\;\times\;10^{-4}[/latex]
(f) [latex]\text{AgBr}(s)\;{\longrightarrow}\;\text{Ag}^{+}(aq)\;+\;\text{Br}^{-}(aq)\;\;\;\;\;\;\;T = 25\;^{\circ}\text{C}\;\;\;\;\;\;\;K_{\text{p}} = 3.3\;\times\;10^{-13}[/latex]
- Calculate the equilibrium constant at 25 °C for each of the following reactions from the value of ΔG° given.
(a) [latex]\text{O}_2(g)\;+\;2\text{F}_2(g)\;{\longrightarrow}\;2\text{OF}_2(g)\;\;\;\;\;\;\;{\Delta}G^{\circ} = -9.2\;\text{kJ}[/latex]
(b) [latex]\text{I}_2(s)\;+\;\text{Br}_2(l)\;{\longrightarrow}\;2\text{IBr}(g)\;\;\;\;\;\;\;{\Delta}G^{\circ} = 7.3\;\text{kJI}[/latex]
(c) [latex]2\text{LiOH}(s)\;+\;\text{CO}_2(g)\;{\longrightarrow}\;\text{Li}_2\text{CO}_3(s)\;+\;\text{H}_2\text{O}(g)\;\;\;\;\;\;\;{\Delta}G^{\circ} = -79\;\text{kJ}[/latex]
(d) [latex]\text{N}_2\text{O}_3(g)\;{\longrightarrow}\;\text{NO}(g)\;+\;\text{NO}_2(g)\;\;\;\;\;\;\;{\Delta}G^{\circ} = -1.6\;\text{kJ}[/latex]
(e) [latex]\text{SnCl}_4(l)\;{\longrightarrow}\;\text{SnCl}_4(l)\;\;\;\;\;\;\;{\Delta}G^{\circ} = 8.0\;\text{kJ}[/latex]
- Calculate the equilibrium constant at the temperature given.
(a) [latex]\text{O}_2(g)\;+\;2\text{F}_2(g)\;{\longrightarrow}\;2\text{F}_2\text{O}(g)\;\;\;\;\;\;\;(T = 100\;^{\circ}\text{C})[/latex]
(b) [latex]\text{I}_2(s)\;+\;\text{Br}_2(l)\;{\longrightarrow}\;2\text{IBr}(g)\;\;\;\;\;\;\;(T = 0.0\;^{\circ}\text{C})[/latex]
(c) [latex]2\text{LiOH}(s)\;+\;\text{CO}_2(g)\;{\longrightarrow}\;\text{Li}_2\text{CO}_3(s)\;+\;\text{H}_2\text{O}(g)\;\;\;\;\;\;\;(T = 575\;^{\circ}\text{C})[/latex]
(d) [latex]\text{N}_2\text{O}_3(g)\;{\longrightarrow}\;\text{NO}(g)\;+\;\text{NO}_2(g)\;\;\;\;\;\;\;(T = -10.0\;^{\circ}\text{C})[/latex]
(e) [latex]\text{SnCl}_4(l)\;{\longrightarrow}\;\text{SnCl}_4(g)\;\;\;\;\;\;\;(T = 200\;^{\circ}\text{C})[/latex]
- Consider the following reaction at 298 K:
[latex]\text{N}_2\text{O}_4(g)\;{\rightleftharpoons}\;2\text{NO}_2(g)\;\;\;\;\;\;\;K_{\text{p}} = 0.142[/latex]What is the standard free energy change at this temperature? Describe what happens to the initial system, where the reactants and products are in standard states, as it approaches equilibrium.
- Under what conditions is [latex]\text{N}_2\text{O}_3(g)\;{\longrightarrow}\;\text{NO}(g)\;+\;\text{NO}_2(g)[/latex] spontaneous?
- Hydrogen sulfide is a pollutant found in natural gas. Following its removal, it is converted to sulfur by the reaction [latex]2\text{H}_2\text{S}(g)\;+\;\text{SO}_2(g)\;{\rightleftharpoons}\;\frac{3}{8}\text{S}_8(s\text{, rhombic})\;+\;2\text{H}_2\text{O}(l)[/latex]. What is the equilibrium constant for this reaction at 298 K? Is the reaction endothermic or exothermic?
- In the laboratory, hydrogen chloride (HCl(g)) and ammonia (NH3(g)) often escape from bottles of their solutions and react to form the ammonium chloride (NH4Cl(s)), the white glaze often seen on glassware. Assuming that the number of moles of each gas that escapes into the room is the same, what is the maximum partial pressure of HCl and NH3 in the laboratory at room temperature? (Hint: The partial pressures will be equal and are at their maximum value when at equilibrium.)
- Carbon dioxide decomposes into CO and O2 at elevated temperatures. What is the equilibrium partial pressure of oxygen in a sample at 1000 °C for which the initial pressure of CO2 was 1.15 atm?
- Acetic acid, CH3CO2H, can form a dimer, (CH3CO2H)2, in the gas phase.
[latex]2\text{CH}_3\text{CO}_2\text{H}(g)\;{\longrightarrow}\;(\text{CH}_3\text{CO}_2\text{H})_2(g)[/latex]The dimer is held together by two hydrogen bonds with a total strength of 66.5 kJ per mole of dimer.
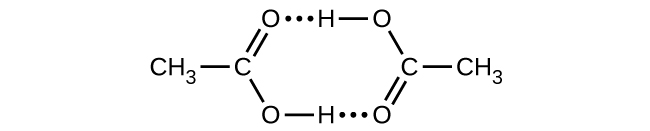
At 25 °C, the equilibrium constant for the dimerization is 1.3 × 103 (pressure in atm). What is ΔS° for the reaction?
- Determine ΔGr° for the following reactions.
(a) Antimony pentachloride decomposes at 448 °C. The reaction is:
[latex]\text{SbCl}_5(g)\;{\longrightarrow}\;\text{SbCl}_3(g)\;+\;\text{Cl}_2(g)[/latex]
An equilibrium mixture in a 5.00 L flask at 448 °C contains 3.85 g of SbCl5, 9.14 g of SbCl3, and 2.84 g of Cl2.
(b) Chlorine molecules dissociate according to this reaction:
[latex]\text{Cl}_2(g)\;{\longrightarrow}\;2\text{Cl}(g)[/latex]
1.00% of Cl2 molecules dissociate at 975 K and a pressure of 1.00 atm.
- Determine the standard molar free energy change, ΔG°f, for the formation of S2−(aq) given that ΔG°f for Ag+(aq) and Ag2S(s) are 77.1 kJ/mole and −39.5 kJ/mole respectively, and the solubility product for Ag2S(s) is 8 × 10−51.
- The evaporation of one mole of water at 298 K has a standard free energy change of 8.58 kJ.
[latex]\text{H}_2\text{O}(l)\;{\rightleftharpoons}\;\text{H}_2\text{O}(g)\;\;\;\;\;\;\;{\Delta}G_{298}^{\circ} = 8.58\;\text{kJ}[/latex](a) Is the evaporation of water under standard thermodynamic conditions spontaneous?
(b) Determine the equilibrium constant, Kp, for this physical process.
(c) By calculating ΔG°vap, determine if the evaporation of water at 298 K is spontaneous when the partial pressure of water, [latex]P_{\text{H}_2\text{O}}[/latex], is 0.011 atm.
(d) If the evaporation of water were always nonspontaneous at room temperature, wet laundry would never dry when placed outside. In order for laundry to dry, what must be the value of [latex]P_{\text{H}_2\text{O}}[/latex] in the air?
- One of the important reactions in the biochemical pathway glycolysis is the reaction of glucose-6-phosphate (G6P) to form fructose-6-phosphate (F6P):
[latex]\text{G}6\text{P}\;{\rightleftharpoons}\;\text{F}6\text{P}\;\;\;\;\;\;\;{\Delta}G_{298}^{\circ} = 1.7\;\text{kJ}[/latex](a) Is the reaction spontaneous or nonspontaneous under standard thermodynamic conditions?
(b) Standard thermodynamic conditions imply the concentrations of G6P and F6P to be 1 M, however, in a typical cell, they are not even close to these values. Calculate ΔGr when the concentrations of G6P and F6P are 120 μM and 28 μM respectively, and discuss the spontaneity of the forward reaction under these conditions. Assume the temperature is 37 °C and ΔS° = 0 J/K (meaning that ΔG° is the same at 25 and 37 °C.)
- When ammonium chloride is added to water and stirred, it dissolves spontaneously and the resulting solution feels cold. Without doing any calculations, deduce the signs of ΔG, ΔH, and ΔS for this process, and justify your choices.
- What happens to ΔG° and ΔG (becomes more negative or more positive) and K and Q (increase or decrease) for the following chemical reactions when the partial pressure of oxygen is increased but temperature is constant?
(a) [latex]\text{S}(s)\;+\;\text{O}_2(g)\;{\longrightarrow}\;\text{SO}_2(g)[/latex]
(b) [latex]2\text{SO}_2(g)\;+\;\text{O}_2(g)\;{\longrightarrow}\;2\text{SO}_3(g)[/latex]
(c) [latex]2\text{HgO}(s)\;{\longrightarrow}\;2\text{Hg}(l)\;+\;\text{O}_2(g)[/latex]
Solutions
- The reaction is nonspontaneous at room temperature.
Above 400 K, ΔG will become negative, and the reaction will become spontaneous. - (a) 465.1 kJ nonspontaneous; (b) −106.86 kJ spontaneous; (c) −53.6 kJ spontaneous; (d) −83.4 kJ spontaneous; (e) −406.7 kJ spontaneous; (f) −154.2 kJ spontaneous
- (a) −1124.3 kJ/mol for the standard free energy change. (b) The calculation agrees with the value because free energy is a state function (just like the enthalpy and entropy), so its change depends only on the initial and final states, not the path between them.
- (a) The reaction is nonspontaneous; (b) Above 438 K or above 165 °C the process is spontaneous.
- (a) 1.5 × 102 kJ; (b) −21.9 kJ; (c) −5.34 kJ; (d) −0.383 kJ; (e) 18 kJ; (f) 71 kJ
- (a) K = 4 × 101; (b) K = 0.05; (c) K = 7 × 1013; (d) K = 1.9; (e) K = 0.04
- In each of the following, the value of ΔG° is not given at the temperature of the reaction. Therefore, we must calculate ΔG° from the values ΔH° and ΔS° and then calculate ΔG° from the relation ΔG° = ΔH° − TΔS°.
(a) K = 1 × 10−13; (b) K = 2.4 × 10−3; (c) K = 1.2 × 104; (d) K = 0.227; (e) K = 16 - The standard free energy change is [latex]{\Delta}G_{298}^{\circ} = -RT\;\text{ln}\;K = 4.84\;\text{kJ/mol}[/latex]. When reactants and products are in their standard states (1 bar or 1 atm), Q = 1. As the reaction proceeds toward equilibrium, the reaction shifts left (the amount of products drops while the amount of reactants increases): Q < 1, and ΔG298 becomes less positive as it approaches zero. At equilibrium, Q = K, and ΔG = 0.
- The reaction will be spontaneous at temperatures greater than 287 K.
- K = 6.4 × 1018
The process is exothermic. - 1.0 × 10−8 atm. This is the maximum pressure of the gases under the stated conditions.
- [latex]x = 1.2\;\times\;10^{-5}\;\text{atm} = P_{\text{O}_2}[/latex]
- ΔS° = −163 J/K = −0.163 kJ/K
- (a) ΔG°r = 22.1 kJ/mol; (b) ΔG°r = 81.1 kJ/mol
- ΔG°f = 92 kJ/mol
- (a) Under standard thermodynamic conditions, the evaporation is nonspontaneous; (b) Kp = 0.031; (c) ΔG°vap = −2.6 kJ/mol, the evaporation of water is spontaneous; (d) [latex]P_{\text{H}_2\text{O}}[/latex] must always be less than Kp or less than 0.031 atm. 0.031 atm represents air saturated with water vapor at 25 °C, or 100% humidity.
- (a) Nonspontaneous as ΔG°298 > 0; (b) ΔG°310 = ΔG°298 since ΔS° = 0 J/K and thus, ΔG° = ΔH° − TΔS° = ΔH° (assumed to be nearly constant over small temperature ranges without changes in state).
ΔGr, 310 = 1.7 × 103 J/mol + (8.314 J/(K mol))(310 K)ln(28/120) = −2.0 × 103 J/mol = −2.0 kJ/mol. The forward reaction to produce F6P is spontaneous under these conditions. - ΔG is negative as the process is spontaneous. ΔH is positive as with the solution becoming cold, the dissolving must be endothermic. ΔS must be positive as this drives the process, and it is expected for the dissolution of any soluble ionic compound.
- (a) Increasing [latex]P_{\text{O}_2}[/latex] will decrease the value of Q and shift the equilibrium toward the products. ΔG therefore becomes more negative. K and ΔG° are unchanged.
(b) Increasing [latex]P_{\text{O}_2}[/latex] will decrease the value of Q and shift the equilibrium toward the products. ΔG therefore becomes more negative. K and ΔG° are unchanged.
(c) Increasing [latex]P_{\text{O}_2}[/latex] will increase the value of Q and shift the equilibrium toward the reactants. ΔG therefore becomes more positive. K and ΔG° are unchanged.

