Unit 2 Kinetics
2.3 Exercises
OpenStax
Section 2.3 Exercises
- Use the data provided to graphically determine the order and rate constant of the following reaction: [latex]\text{SO}_2\text{Cl}_2\;{\longrightarrow}\;\text{SO}_2\;+\;\text{Cl}_2[/latex]
Time (s) 0 5.00 × 103 1.00 × 104 1.50 × 104 [SO2Cl2] (M) 0.100 0.0896 0.0802 0.0719 Time (s) 2.50 × 104 3.00 × 104 4.00 × 104 [SO2Cl2] (M) 0.0577 0.0517 0.0415 - What is the half-life for the first-order decay of phosphorus-32? ([latex]_{15}^{32}\text{P}\;{\longrightarrow}\;_{16}^{32}\text{S}\;+\;\text{e}^{-})[/latex]. The rate constant for the decay is 4.85 × 10−2 day−1.
- Some bacteria are resistant to the antibiotic penicillin because they produce penicillinase, an enzyme with a molecular weight of 3 × 104 g/mol that converts penicillin into inactive molecules. Although the kinetics of enzyme-catalyzed reactions can be complex, at low concentrations this reaction can be described by a rate equation that is first order in the catalyst (penicillinase) and that also involves the concentration of penicillin. From the following data: 1.0 L of a solution containing 0.15 µg (0.15 × 10−6 g) of penicillinase, determine the order of the reaction with respect to penicillin and the value of the rate constant.
[Penicillin] (M) Rate (mol/L/min) 2.0 × 10−6 1.0 × 10−10 3.0 × 10−6 1.5 × 10−10 4.0 × 10−6 2.0 × 10−10 - There are two molecules with the formula C3H6. Propene, [latex]\text{CH}_3\text{CH}=\text{CH}_2[/latex], is the monomer of the polymer polypropylene, which is used for indoor-outdoor carpets. Cyclopropane is used as an anesthetic:
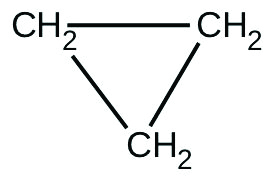
When heated to 499 °C, cyclopropane rearranges (isomerizes) and forms propene with a rate constant of 5.95 × 10−4 s−1. What is the half-life of this reaction? What fraction of the cyclopropane remains after 0.75 h at 499.5 °C?
- Suppose that the half-life of steroids taken by an athlete is 42 days. Assuming that the steroids biodegrade by a first-order process, how long would it take for [latex]\frac{1}{64}[/latex] of the initial dose to remain in the athlete’s body?
- Nitroglycerine is an extremely sensitive explosive. In a series of carefully controlled experiments, samples of the explosive were heated to 160 °C and their first-order decomposition studied. Determine the average rate constants for each experiment using the following data:
Initial [C3H5N3O9] (M) 4.88 3.52 2.29 1.81 5.33 4.05 2.95 1.72 t (s) 300 300 300 300 180 180 180 180 % Decomposed 52.0 52.9 53.2 53.9 34.6 35.9 36.0 35.4
Solutions
- Plotting a graph of ln[SO2Cl2] versus t reveals a linear trend; therefore we know this is a first-order reaction:

k = 2.20 × 105 s−1 - 14.3 d
- The reaction is first order.
k = 5.0 × 10−5 min−1 - Half-life = 1.16 × 103 s1; 20% remains
- 252 days
-
[A]0 (M) k × 103 (s−1) 4.88 2.45 3.52 2.51 2.29 2.54 1.81 2.58 5.33 2.35 4.05 2.44 2.95 2.47 1.72 2.43

