Unit 1 Gases
1.2 Exercises
OpenStax
Section 1.2 Exercises
- Explain how the volume of the bubbles exhausted by a scuba diver change as they rise to the surface, assuming that they remain intact.
- An alternate way to state Avogadro’s law is “All other things being equal, the number of molecules in a gas is directly proportional to the volume of the gas.” (a) What is the meaning of the term “directly proportional?” (b) What are the “other things” that must be equal?
- A spray can is used until it is empty except for the propellant gas, which has a pressure of 1344 torr at 23 °C. If the can is thrown into a fire (T = 475 °C), what will be the pressure in the hot can?
- A 2.50-L volume of hydrogen measured at –196 °C is warmed to 100 °C. Calculate the volume of the gas at the higher temperature, assuming no change in pressure.
- A weather balloon contains 8.80 moles of helium at a pressure of 0.992 atm and a temperature of 25 °C at ground level. What is the volume of the balloon under these conditions?

Source: OpenStax Chemistry - How many moles of gaseous boron trifluoride, BF3, are contained in a 4.3410-L bulb at 788.0 K if the pressure is 1.220 atm? How many grams of BF3?
- How many grams of gas are present in each of the following cases?
(a) 0.100 L of CO2 at 307 torr and 26 °C
(b) 8.75 L of C2H4, at 378.3 kPa and 483 K
(c) 221 mL of Ar at 0.23 torr and –54 °C
- A cylinder of medical oxygen has a volume of 35.4 L, and contains O2 at a pressure of 151 atm and a temperature of 25 °C. What volume of O2 does this correspond to at normal body conditions, that is, 1 atm and 37 °C?
- A 20.0-L cylinder containing 11.34 kg of butane, C4H10, was opened to the atmosphere. Calculate the mass of the gas remaining in the cylinder if it were opened and the gas escaped until the pressure in the cylinder was equal to the atmospheric pressure, 0.983 atm, and a temperature of 27 °C. Which given information was not actually needed to solve this problem?
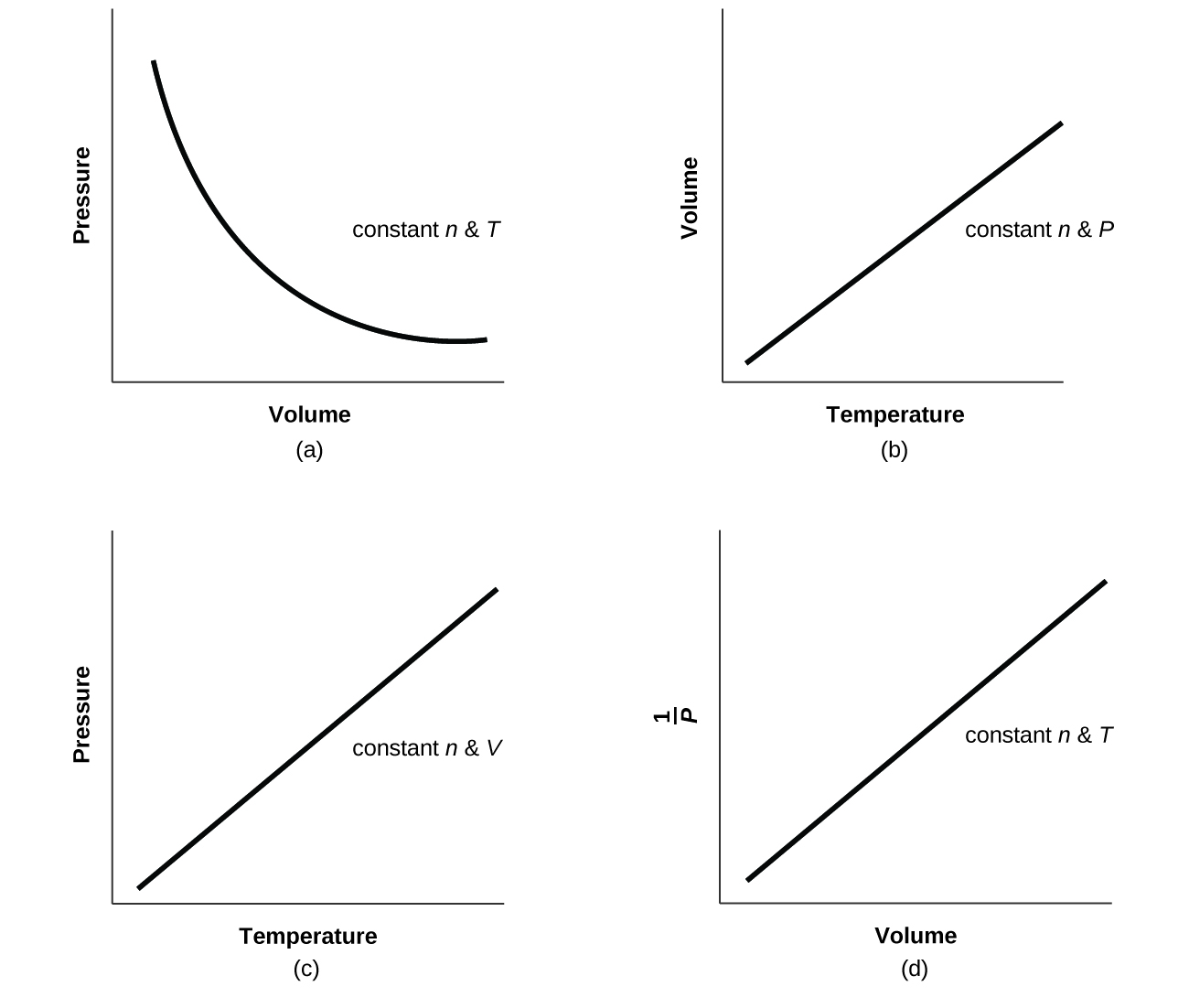
- For a given amount of gas showing ideal behavior, draw labeled graphs of:
(a) the variation of P with V
(b) the variation of V with T
(c) the variation of P with T
(d) the variation of [latex]\frac{1}{P}[/latex] with V
- The effect of chlorofluorocarbons (such as CCl2F2) on the depletion of the ozone layer is well known. The use of substitutes, such as CH3CH2F(g), for the chlorofluorocarbons, has largely corrected the problem. Calculate the volume occupied by 10.0 g of each of these compounds at STP:
(a) CCl2F2(g)
(b) CH3CH2F(g)
- A balloon that is 100.21 L at 21 °C and 0.981 atm is released and just barely clears the top of Mount Crumpet in British Columbia. If the final volume of the balloon is 144.53 L at a temperature of 5.24 °C, what is the pressure experienced by the balloon as it clears Mount Crumpet?
- If the volume of a fixed amount of a gas is tripled at constant temperature, what happens to the pressure?
Solutions
- As the bubbles rise, the pressure decreases, so their volume increases as suggested by Boyle’s law.
- (a) The number of particles in the gas increases as the volume increases. (b) temperature, pressure.
- 3.40 × 103 torr
- 12.1 L
- 217 L
- 8.190 × 10–2 mol; 5.553 g
- (a) 7.24 × 10–2 g
(b) 23.1 g
(c) 1.5 × 10–4 g - 5561 L
- 46.4 g; the mass of butane was not needed to solve the problem
- For a gas exhibiting ideal behavior:
- (a) 1.85 L CCl2F2
(b) 4.66 L CH3CH2F - 0.644 atm
- The pressure decreases by a factor of 3.

